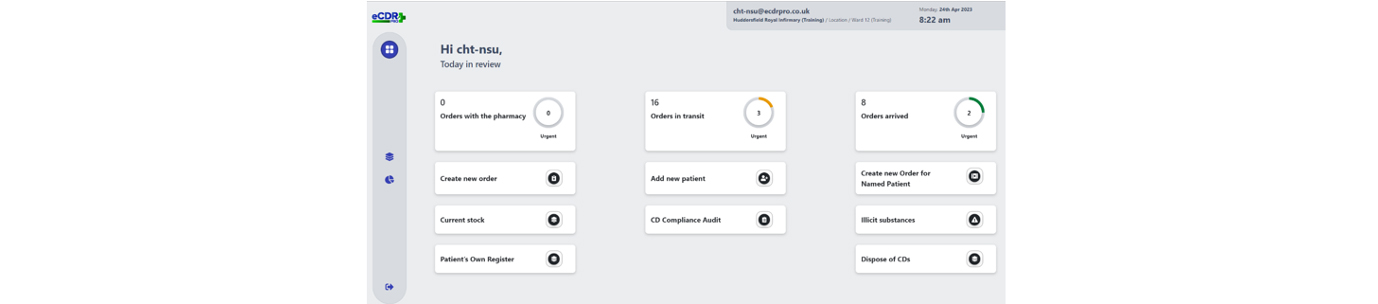
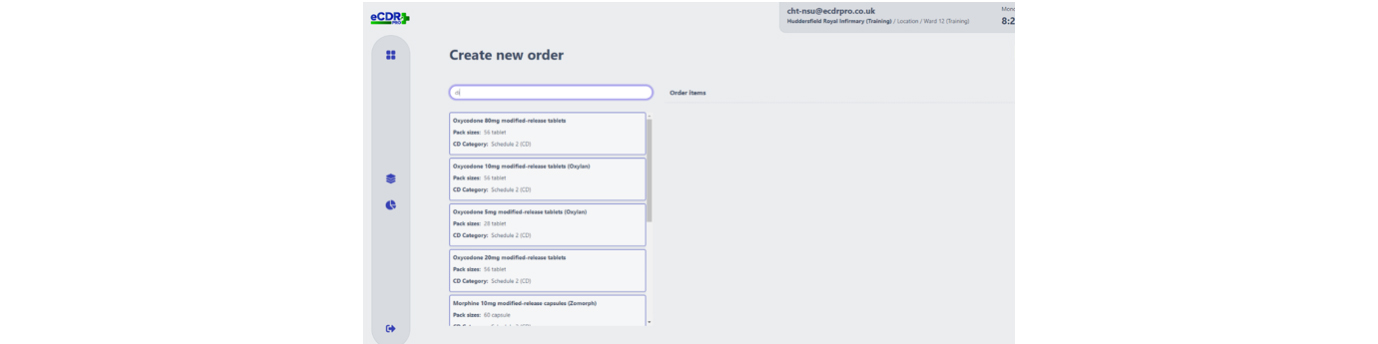
Going paperless or increasing and improving digitisation - whichever way you want to describe it - is an objective firmly embedded on the NHS agenda, and one that has been grasped with enthusiasm by leaders at the Calderdale and Huddersfield NHS Foundation Trust (CHFT), which has added another digital innovation, a first-of-type electronic controlled drugs register, to its growing list of digital achievements.
Documentation in CD registers requires great attention to detail. It is illegal to cross out or use correction-type products, but in clinical areas, where multiple doses are administered by staff working in very busy environments, it was understandable that documentation errors might happen.
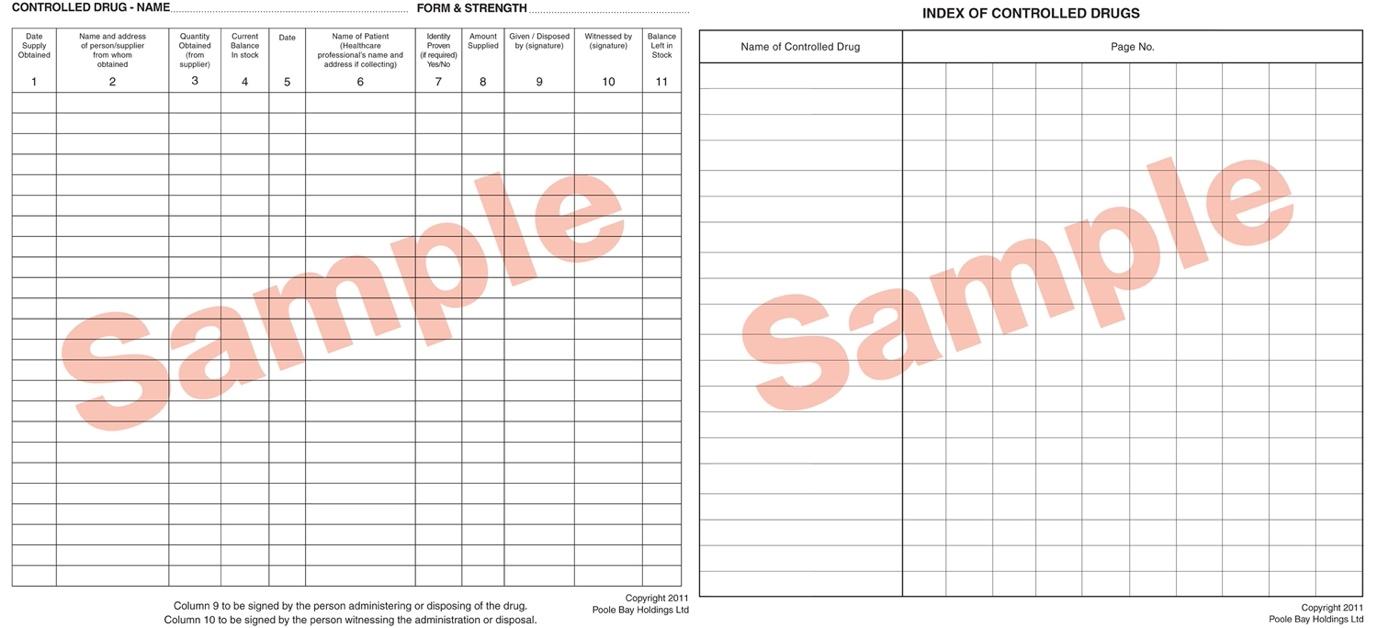
Recognising that any handwritten process was open to human error, crossings out or miscalculations, and to avoid such errors, CHFT sought a digital solution to mitigate these issues and offer time saving benefits at the two hospitals it runs in West Yorkshire – Huddersfield Royal Infirmary and Calderdale Royal Hospital in Halifax.
Penny Daynes, CHFT’s Lead Pharmacist for Operations - Medicines Management and Procurement, says:
“We’ve been fortunate to find the right software development partner to work with. It is new and unique, and we don’t think any other trust has managed to implement what we have done.”
Good practice combined with legal compliance
Controlled drugs (CDs) are an essential element of modern clinical care. They include medications such as morphine and oxycodone that are used in a wide variety of clinical treatments, such as the relief of acute and chronic pain, end-of-life treatments or as part of the treatment for substance misuse.
Other medicines such as anxiolytics to relieve anxiety, sleeping pills, steroids and growth hormones are also designated as CDs, although these are subject to less stringent controls under the Home Office’s Misuse of Drugs legislation.
Clear oversight of controlled drug supply and administration is a legislative requirement that all NHS organisations must abide by. Robust documentation and recording for every dose supplied and administered is subject to strict documentation rules that are not merely good practice, but necessary for the legalities of controlled drug usage.
The search for a digital solution has resulted in a bespoke, first-of-type system developed over two years in partnership with a software company called Solidsoft Reply, which specialises in developing digital healthcare solutions for patient safety and closed-loop medicine management.
Saving time and improving care
Three years after the search began, the electronic CD register, now known as the eCDR-PRO, is live in 12 clinical areas and one inpatient pharmacy, with training underway in the remaining clinical areas. At the time of writing, the aim is to have the eCDR-PRO fully implemented by late summer 2023.
In addition to improved legal compliance through better recording, the other benefits include significant time savings on auditing and reviewing for pharmacy and ward staff and trust managers.
Using the eCDR-PRO now allows for real time auditing for stock reconciliation and reduces the time required by ward and pharmacy staff to audit stock quantities. It is also being used as a stock management tool in times of drug shortages, allowing an overview of stock on all wards where previously the EMIS pharmacy system only showed stock available in pharmacy, not on the wards or in theatres.
Scrapping the handwritten register system is also saving time. Previously, the books were hand delivered to pharmacy by nursing staff along with a controlled drug storage bag to request top up stocks for their ward areas.
Only registered healthcare professionals are allowed to order CD stock and with approximately 50 requisitions processed each day, it meant 50 delivery visits to pharmacy. If each trip took approximately five minutes, that equated to more than four hours of nursing time daily, totalling 1,400 hours a year of ‘wasted’ time that can then be spent on nursing care.
The eCDR-PRO now allows clinical areas to request CDs from pharmacy for stock or for named patient use.
More positive impacts of the eCDR-PRO
In addition to the benefits outlined above, trust leaders have recognised more gains brought about by the implementation of the eCDR-PRO:
- Orders are sent overnight and are ready as soon as pharmacy opens saving 10 to 15 minutes each time an order is placed.
- Orders are accurate - this saves pharmacy time resolving unclear requests and also stops orders from unapproved staff.
- Order processing and receipt back to the ward is quicker - reducing delayed or omitted doses.
- Actual stock balance is checked at every administration and the second check is recorded as a mandatory field where appropriate.
- The system instantly highlights discrepancies.
- Audit completion is now quicker.
- Time taken to stock check has also reduced.
- Automatically reports if there are deviations from the stock balance in the CD register.
- Automatically flags concerns to appropriate staff so they can act immediately on any anomalies.
Finding the right collaborator
Market analysis and research showed that an electronic CD register with the capabilities necessary for hospital use did not exist at the time of the review.
However, throughout the course of the research, the trust’s medication safety team engaged in dialogue with Solidsoft Reply, which offered to develop a bespoke product that could then be used by other trusts.
With the business case signed off, funding secured jointly from CHFT’s capital expenditure group and the government’s Digital Aspirant Fund, and Solidsoft’s proven track record of development, the partnership was forged.
Lis Street, CHFT’s Clinical Director of Pharmacy and CDAO (Controlled Drugs Accountable Officer) says:
“It was a whole new world for us in pharmacy working with a software developer. The terminology they use was very different from our normal pharmacy world and hopefully both sides learnt a lot from each other during this development journey.
“Originally, we hoped there would be an off-the-shelf product to meet our needs. As we worked with Solidsoft we could see the potential for an acute trust system that would in addition to meeting the legalities of controlled drugs recording, could also save our staff time, have better audit functionality and also be used for on-call pharmacist queries.”
Some of the work was done during the Covid-19 pandemic, which illustrated a further benefit the eCDR-PRO could bring. It could – and has – reduced physical interaction between a clinical area treating highly infectious diseases and other parts of the hospitals by removing the need to transfer the CD order books from one area to another.
Key role for The Health Informatics Service (THIS)
CHFT is host to The Health Informatics Service (THIS), which has been at the cutting edge of NHS digitisation since its inception more than 20 years ago. Its work with CHFT is helping to make it one of the most ‘digitally mature’ and ‘paper light’ trusts in the UK.
THIS most often becomes involved in a project from the outset and typically facilitates 40-50 jobs for its host trust and other clients in the healthcare sector at any one time.
Its Project Management Office (PMO) employs nine project managers of varying seniority, plus a project support officer, who provide support and assistance with the writing of business cases and then project management.
All project managers employed by THIS are Prince2 practitioners and base their work on the seven processes of that methodology:
- Starting up a project
- Directing a project
- Initiating a project
- Controlling a stage
- Managing product delivery
- Managing stage boundaries
- Closing the project
However, while most projects conform to Prince2, THIS can tailor projects according to size and complexity and will introduce agile methodology where necessary.
In this case, however, the eCDR-PRO project business case was already approved by the trust when it asked for a THIS project manager to be assigned to manage and deliver the project.
That role has been fulfilled by Susan Lacey since January 2022, and she has led a wide-ranging team of THIS expertise, that is supporting the eCDR-PRO project. They are:
- Network team – for the initial setting up of the Azure/AD directory for user accounts.
- Information Governance Team – to review and sign off supplier DTAC/DPIA
- Central deployment team – for the creation and population of eCDR-PRO group folder accounts from the trust’s AD.
- Service desk – to handle any eCDR-PRO access/account issues following the handover to BAU (business as usual).
An integration specialist is also looking at assimilating the eCDR-PRO with CHFT’s Oracle Millennium EPR.
Putting the building blocks in place – with and without Covid
The eventual easing of Covid restrictions alleviated limitations on the system build by allowing for increased testing by the project team and other stakeholders.
Penny Daynes:
“(Because of Covid) everything was done remotely to begin with, and we had to make that work, but it was much more productive when Solidsoft had people here on site engaging with staff in person. So, that's a lesson we've learned, that it's always much better to do things face-to-face where possible.”
The build was done in two testing phases with iterative input by clinical staff, including the trust’s Medication Safety Officer and Medicines Management Nurse, to achieve desired functionality based on pharmacy and nursing workflows. The team worked collaboratively to deliver a solution that is user-friendly and optimised for secondary care usage in pharmacy and on the wards, theatres and departments.
Penny Daynes:
“It was like building a jigsaw. You get one piece right, you then you build the other piece and then put them together. We focused on one workflow at a time, so we’d look at the nursing side of things and then at the pharmacy aspect to bring it all together. We called each building phase a sprint of functionality until they were all completed and the whole system was built.”
Names and identifiers of the drugs needed to be input on to the eCDR-PRO were gleaned from CHFT’s EMIS hospital pharmacy stock control system, and all information is compliant with dm+d (Dictionary of medicines and devices), which is a glossary of descriptions and codes for medicines and devices used across the NHS.
Aiming for integration and interoperability
As the latest addition to CHFT’s growing list of digital innovations, a longer term aspiration for the eCDR-PRO is to create integration and interoperability with other digital assets implemented by the trust.
The first is to integrate automated electronic medicine cabinets in use in some areas of the trust, such as local emergency departments, intensive care units and maternity wards, into the system.
The cabinets – essentially large vending machines - use biometric information (fingerprints) to allow staff to access medications – replacing the traditional locked cupboard and the need to find physical keys.
Lis Street:
“At the moment the eCDR-PRO is a standalone system because we wanted a system that is trust wide, not just available to areas who have an automated cabinet. Everybody can access it regardless of where they work, whether they have a conventional CD cupboard or an electronic cabinet, and we have visibility across the whole hospital. It’s one system with one set of SOPs (Standard Operating Procedures) setting future standards.”
Both CHFT and Solidsoft Reply see the eCDR-PRO as a system that could easily be rolled out in other NHS trusts to replace the traditional register, and indeed, other trusts have made enquiries about it already.
The project has also garnered interest at the West Yorkshire controlled drugs intelligence network attended by CD accountable officers from the region’s hospitals, hospices and community trust providers and representatives from the CQC and the police.
Lis Street:
“I was asked to go along to the group and tell our story. Many organisations have the same issues but how widely the eCDR-PRO is adopted will depend on finances, I think. I don’t think it’s people not seeing the benefits, it’s whether they can afford it, how it fits with their priorities and when the appropriate pots of digital funding become available.”
Contact THIS
As well as collaborating with our host trust, THIS project managers can work across the entire healthcare sector such as GP practices, laboratories, hospices, care charities and NHS trusts. Our clients include London’s Great Ormond Street Hospital, and hospital trusts in Southampton, Oxford, Cambridge, Nottingham, Derby, Birmingham, Liverpool, Manchester, Middlesbrough, North Tees & Hartlepool, Newcastle Upon Tyne, Edinburgh, Lanarkshire and Glasgow.
If you’d like to collaborate and innovate with THIS on a project in your healthcare organisation, get in touch.

Subscribe to Informatics Insights & Advice
Take advantage of the latest news and information from The Health Informatics Service. Read about our innovative work with healthcare clients across the UK and get our expert insights and recommendations to help and inspire your work.